 GETTING TO KNOW THE TWO MAJOR CATIONS
GETTING TO KNOW THE TWO MAJOR CATIONS
CALCIUM General "Warm Up" -
To commence our introduction to some of the elements we will start with calcium. It is considered first
due to its sheer volume in a healthy soil. The soil requires 60-70% available Calcium base saturation
percentage in a good soil.
Calcium loosens your soils, but if there is too much Calcium surface sealing can result as all the
fine particles mass together. Calcium comes in many forms, but we will just touch on a few main forms.
Rock phosphate [ Ca + P ]
What a wonderful product, it has both calcium and phosphorous and has served humanity well for hundreds
of years. It is still available and there tends to be the finely crushed stuff or a product like small
balls. Most of the good rock phosphate i.e. "A" grade rock has been used up and caution must be shown
in finding out if there are any nasties like cadmium or fluoride in them. This problem should not be a
major concern for companies that blend rocks. But just check anyway and avoid rocks that have high levels
where economically possible.
Limestone [ Ca + C ]
Lime is calcium carbonate and it adds carbon to the soil which is a bonus.
Heavily Liming a soil will remove and lower other elements that you have tried to put in the soil
like potassium, copper, zinc etc so DON'T OVER DO LIME. It can cost twice as much to fix a problem of
over liming than to add lime. Working lime into the soil is always best, but don't let that stop you
liming pasture. Lime takes time to become available 3-12+ months so plan ahead.
There are innumerable documented examples of the benefits of liming the soil that date back prior to
Roman times. In 'Soils an Australian perspective' to use their words "Unique features of the Australian
approach have been .... although the soils have often been acid, the use of lime has been rare" [CSIRO,
p.603, 1993]. Even Victorian agriculture with close access to lime has followed the Australian trend and
has a low appreciation of the benefits of lime due in part to a poor and outdated understanding of soil
science and a reluctance on behalf of soil scientists to change from their current paradigms.
Lime could have had a bad image because it is relatively cheap.
It has been the cheap man's fertiliser when he could not afford the good stuff i.e. super.
Dolomite [ Ca + Mg ]
Dolomite = Calcium and Magnesium.
DON'T USE DOLOMITE ON HIGH MAGNESIUM SOILS.
Dolomites tend to be a harder product than some limestone. As a result it is often not so easily crushed
and this may result in a slower release time. So don't expect quick results to overcome a magnesium problem.
Gypsum [ Ca + S ]
A good product if used in the right place.
Remember it is good for raising sulphur levels as well.
If your calcium levels are low, calcium in the form of lime will raise your soil levels higher than the use
of gypsum. Gypsum does not always give you a gross increase in calcium.
CATION BALANCE
The Albrecht Soil balance system has identified general desired ratios for the five major cations.
This detail is provided on the Base Saturation Percentage report.
|
A healthy productive soil should have about:- |
68% Calcium ++
12% Magnesium ++
12% Hydrogen
2-5% Potassium
<1% Sodium
|
|
SUMMARY: CATIONS
- We have briefly introduced cations and started to understand more about their characteristics
which are going to influence your bank account so much.
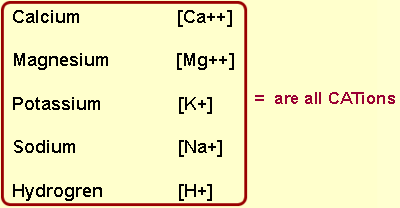
These elements are Tigers supporters being CAT[ion]s
They all have a positive charge
to them [+]
Other cations include: Iron [Fe++], Copper [Cu++], Zinc[Zn++] & Manganese [Mn++].
We will meet these elements later on and they will be described as "metallic"
elements as they are all metals, e.g. Copper pipe
The information contained in this publication has been formulated in good faith, the contents do
not take into account all the factors which need to be considered before putting that information
into practice. Accordingly, no person should rely on anything contained herein as a substitute for
specific professional advice.
S.O.S. Rev 9.2 All rights reserved.
Contact: www.healthyag.com © Gwyn Jones 2001
On to page 4
PAGE
1 - 2 -
3 - 4
|

![]() Home
Home![]()
![]() History
History![]()
![]() Catalogue
Catalogue![]()
![]() Courses
Courses![]()
![]() Top Ten
Top Ten![]()
![]() Links
Links![]()
![]() Site Map
Site Map![]()
![]() Contact
Contact


